BioNTech has clinched an agreement with Rentschler Biopharma SE, a global contract development and manufacturing organization (CDMO) for large-scale manufacturing of its Covid-19 mRNA vaccine candidate.
As part of the agreement, Rentschler will act as BioNTech’s (BNTX) CDMO partner. Initially, Rentschler will be responsible for key aspects of cGMP (current good manufacturing practice) drug substance manufacturing of BNT162b2, the mRNA-based vaccine against SARS-CoV-2 that is being developed by BioNTech in collaboration with Pfizer and is currently in a global Phase 3 clinical trial.
Under the terms of the agreement, Rentschler will take care of downstream processing to produce highly purified drug substance. This means, Rentschler said, that process and product-related impurities will be effectively removed from the intermediate pool, which has been previously derived from mRNA synthesis. Rentschler noted this is an important step in ensuring the safety and tolerability of a vaccine for use in humans, while at the same time maximizing the amount of mRNA harvested from the initial production process.
In addition to large-scale production services for the Covid-19 vaccine candidate, the agreement also covers small-batch manufacturing of BioNTech’s other RNA programs for use in clinical trials.
The BNT162b2 vaccine candidate is based on BioNTech’s proprietary mRNA technology and supported by Pfizer’s global vaccine development and manufacturing capabilities. It encodes an optimized SARS-CoV-2 full-length spike glycoprotein (S), which is a target of virus-neutralizing antibodies.
The vaccine candidate is currently being evaluated in a global Phase 3 study ongoing at more than 120 clinical sites worldwide including the US, Brazil, South Africa, and Argentina. To date, the trial has enrolled about 37,000 participants with more than 28,000 having received their second vaccination.
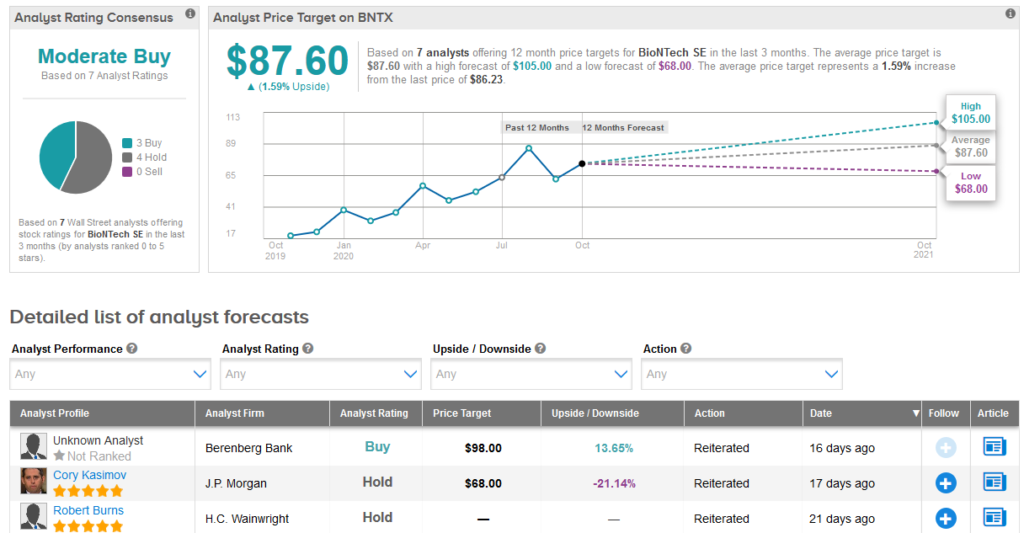
With BioNTech shares up a whopping 155% so far this year, analysts now forecast a modest 1.6% upside potential over the coming year setting the average price target at $87.60.
H.C. Wainwright analyst Robert Burns last month reiterated a Hold rating on the stock.
“Despite the encouraging preclinical and clinical results seen for BNT162b2 thus far, we cannot predict the vaccine’s commercial potential with certainty, given the continually evolving epidemiology of Covid-19, and the rapidly changing nature of the competitive landscape,” Burns wrote in a note to investors. “Moreover, there are growing concerns regarding the logistical cold-storage supply chain that can deliver mRNA-based vaccines without letting them become warm and thus ineffective.”
The rest of the Street is cautiously optimistic on the stock with a Moderate Buy analyst consensus. (See BioNTech stock analysis on TipRanks).
Related News:
Gilead Inks EU Supply Deal For 500,000 Remdesivir Doses
Regeneron Files For Emergency Use Nod Of Covid-19 Antibody Cocktail; Shares Rise
Eli Lilly Files For FDA Emergency Use Of COVID-19 Antibody Treatment









